From Aromatics to Aldehydes: Mastering the Gattermann-Koch Transformation
Introduction:
Chemical transformations are the cornerstone of modern synthesis techniques, enabling the creation of diverse organic compounds. The Gattermann-Koch reaction, a formylation process, holds a significant place in the realm of organic chemistry. This article explores the mechanics of the Gattermann-Koch reaction, its formylation process, reagents involved, and exemplifies its application through real-world examples.
The Gattermann-Koch Reaction:
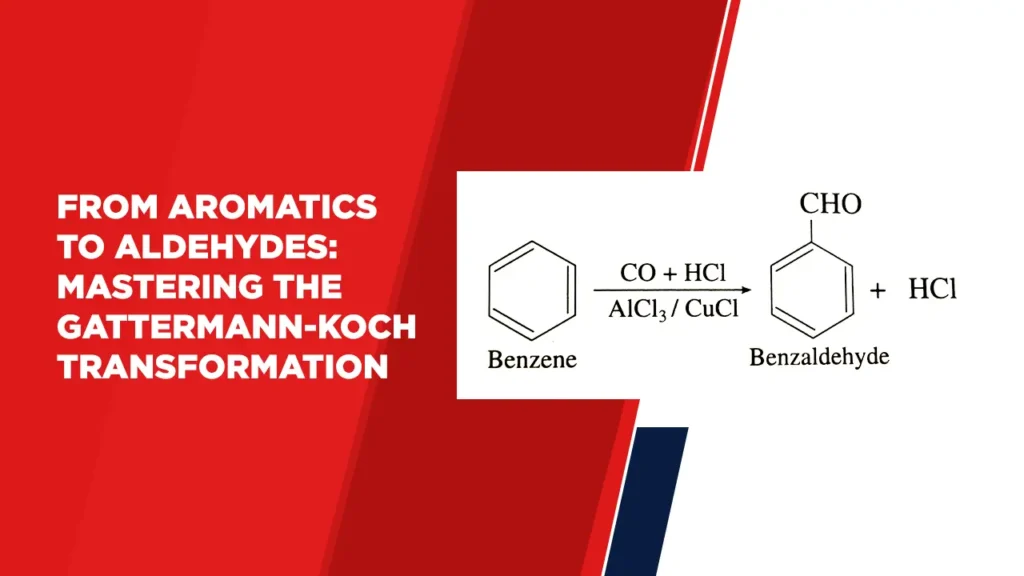
The Gattermann-Koch reaction is a method used for the synthesis of aldehydes from aromatic compounds. It allows the selective introduction of a formyl group (CHO) onto the aromatic ring, leading to the conversion of various aromatic compounds into aldehydes.
Mechanism of Gattermann-Koch Reaction:
The Gattermann-Koch reaction follows a two-step mechanism:
Formation of Diazonium Salt:
The aromatic compound reacts with hydrochloric acid (HCl) and sodium nitrite (NaNO2) to form a diazonium salt intermediate.
Formylation with Hydrogen Cyanide (HCN):
The diazonium salt is then treated with hydrogen cyanide (HCN), which is usually dissolved in the presence of copper(I) chloride (CuCl) or cuprous cyanide (CuCN). The reaction leads to the replacement of the diazonium group with a formyl group (-CHO), resulting in the formation of the aldehyde.
Reagents in Gattermann-Koch Reaction:
The key reagents involved in the Gattermann-Koch reaction are:
Sodium Nitrite (NaNO2):
Used to convert the aromatic compound into a diazonium salt.
Hydrogen Cyanide (HCN): Provides the formyl group for the formylation reaction.
Copper(I) Chloride (CuCl) or Cuprous Cyanide (CuCN):
Catalysts that facilitate the reaction between the diazonium salt and HCN.
Application and Examples:
The Gattermann-Koch reaction finds utility in the synthesis of various aromatic aldehydes. For example, the reaction can be used to convert benzene into benzaldehyde, or to formylate anisole to yield anisaldehyde. These aldehydes serve as versatile intermediates in the production of pharmaceuticals, fragrances, and specialty chemicals.
Conclusion:
The Gattermann-Koch reaction stands as a testament to the creativity and innovation within organic chemistry. By harnessing the power of reagents and reaction mechanisms, chemists can transform simple aromatic compounds into valuable aldehydes. From pharmaceuticals to flavoring agents, the aldehydes synthesized through this reaction have a broad range of applications that impact industries and improve our daily lives. As chemical understanding evolves, the Gattermann-Koch reaction continues to contribute to the expansion of the synthetic toolbox in the pursuit of novel compounds and groundbreaking discoveries.