Revolutionizing Science: How Tension Is Reshaping Our Understanding of the Universe
Introduction: Unraveling the Mysteries of Surface Tension
Surface stress is a fascinating phenomenon that affects our everyday lives more than we realize. From the way water coats a car windshield to the delicate balance of forces that allows some insects to walk on water, tension plays an important role. In this article, we will delve deeper into the definition of surface tension, explore the surface tension formula, and explore the diverse applications of this captivating concept.
Defining Surface Tension
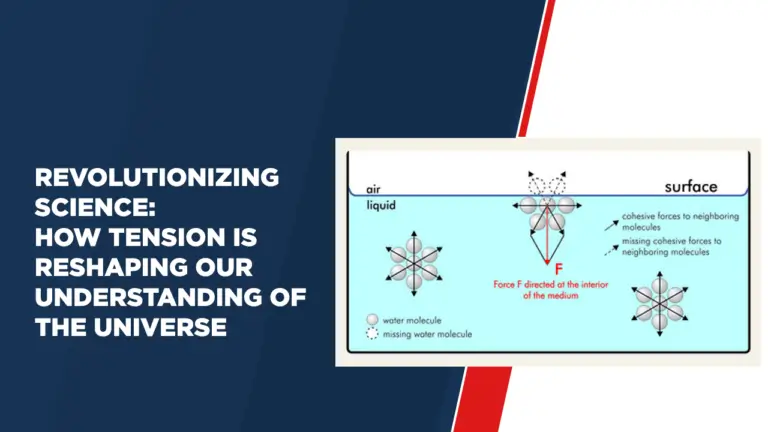
Surface tension, in its simplest form, is the force that exists on the surface of a fluid, causing it to behave like a stretched elastic sheet. This is the result of cohesive forces between liquid molecules. Imagine a trampoline stretched tightly, where each molecule in the liquid pulls inward across the surface like a tiny spring. This force creates tension while trying to reduce the surface area of the liquid.
The Surface Tension Formula
To quantify this concept, we use the surface tension formula:
T = (F / L)
Where:
– T represents the surface tension
– F is the force acting perpendicular to the surface
– L denotes the length along which the force is acting
This formula captures the essence of surface tension, explaining why liquid droplets form spherical shapes, as they reduce surface area to reduce tension.
Applications of Surface Tension
Capillary action:
A notable application of surface tension is capillary action, which allows liquids to flow against gravity in narrow spaces, such as water rising in a narrow tube. This phenomenon plays an important role in plant biology, as it helps transport water and nutrients from the roots to the leaves.
Survival of insects:
Surface tension enables some insects, such as water striders, to defy gravity by walking on water. The insects’ legs distribute their weight over the surface of the water, preventing them from breaking.
Medicine and Biology:
Surface tension aids in various medical and biological processes. For example, it is important for the function of the alveoli in our lungs, where surface tension helps keep the air sacs open for efficient gas exchange. Additionally, it also affects the behavior of cell membranes.
What Is Surface Tension in Practical Terms?
In practical terms, surface tension explains why liquid droplets form the way they do. When you pour water on a surface, it rises because the water molecules on the surface are more attracted to each other than those on the surface below. Understanding surface tension is important in a variety of industries, from pharmaceuticals to materials science, where it affects processes such as emulsion formation and coating of surfaces.
Conclusion: Embracing the Tension
Surface tension, though often unnoticed, is a fundamental force that shapes our world. By understanding its definition, discovering the surface tension formula, and appreciating its applications, we can gain a deeper understanding of the complex interactions that govern the behavior of fluids. This knowledge not only enriches our scientific understanding but also paves the way for innovative solutions in fields ranging from engineering to biology. So, the next time you see water droplets rising up, remember that surface tension is at work, quietly influencing the world around us.