Demystifying Markovnikov’s Rule: Predicting Addition Reactions With Precision
Introduction:
In the intricate realm of organic chemistry, Markovnikov’s Rule stands as a guiding principle that illuminates the outcomes of addition reactions involving unsymmetrical molecules. Formulated by Russian chemist Vladimir Markovnikov in the 19th century, this rule is a cornerstone concept that offers profound insights into regioselectivity, a critical aspect of chemical reactions. By grasping the essence of Markovnikov’s Rule, chemists can foretell how atoms or groups align during an addition reaction, facilitating the creation of specific molecular structures.
Understanding Markovnikov’s Rule:
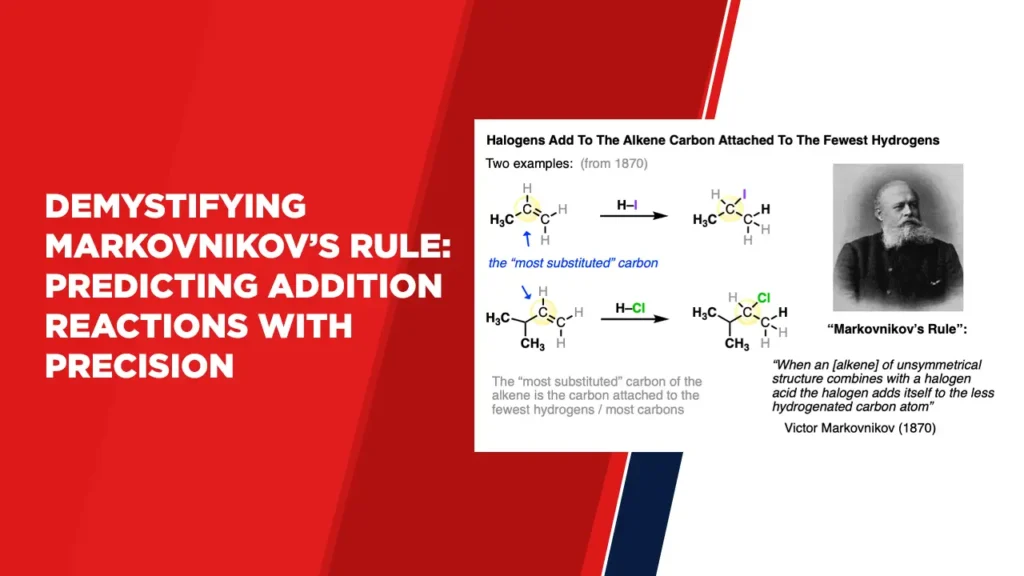
Markovnikov’s Rule revolves around the concept of regioselectivity, determining which carbon atom in a multiple bond structure attracts an added atom or group. Specifically, it predicts that during an addition reaction to a carbon-carbon double or triple bond, the hydrogen atom will attach itself to the carbon atom with fewer hydrogen atoms. Simultaneously, the other atom or group will bond with the carbon atom carrying more hydrogen atoms.
Application of Markovnikov’s Rule:
The utility of Markovnikov’s Rule is most evident in reactions involving hydrogen halides, such as HCl or HBr, and alkenes. When these molecules undergo addition, the hydrogen atom from the hydrogen halide bonds with the carbon atom that possesses fewer alkyl groups, adhering to the principle outlined by Markovnikov’s Rule. This leads to the formation of a carbocation intermediate, which ultimately dictates the outcome of the reaction.
Rationale for Stability:
The crux of Markovnikov’s Rule stems from the stabilization of carbocations. A carbon atom bearing more alkyl groups experiences an influx of electrons due to the electron-donating nature of the alkyl groups, rendering it more stable. On the contrary, a carbon atom with fewer alkyl groups is less electron-rich and thus less stable. The addition of a hydrogen atom to the electron-deficient carbon atom results in the generation of a more stable carbocation, favoring the reaction.
Limitations and Anti-Markovnikov Behavior:
While Markovnikov’s Rule is a guiding principle, exceptions and deviations can arise. In certain circumstances involving radicals or specific catalytic conditions, the reaction may exhibit anti-Markovnikov behaviour. Here, the hydrogen atom attaches to the carbon atom with more alkyl groups, subverting the conventional expectations. Such exceptions underscore the complexity of chemical reactions and the dynamic interplay between various factors.
Conclusion:
Markovnikov’s Rule, a cornerstone in the realm of organic chemistry, empowers scientists to predict and comprehend the outcomes of addition reactions. Its ability to unravel the regioselectivity of reactions enhances the precision and control chemists possess when designing syntheses and manipulating molecular structures. As research advances and our understanding deepens, Markovnikov’s Rule stands as a testament to the elegance and predictive power of fundamental principles, contributing to the ongoing evolution of chemistry and its practical applications.